Optical Hallmarks of Aggressive Clones Within Oral Field Cancerization
Funding Agency:
National Cancer Institute
Collaborators:
Rebecca Richards-Kortum (Rice), Ann Gillenwater (M.D. Anderson Cancer Center), Nadarajah Vigneswaran (University of Texas School of Dentistry)
Overview:
Oral cancer is the 6th most common cancer worldwide. Despite the easy accessibility of the oral cavity for screening, oral cancer has one of the lowest five-year survival rates of all cancers. Oral cancer is thought to arise as a result of field cancerization, where, often in response to tobacco and alcohol exposure, wide areas of the mucosal surface develop subclinical carcinogenetic changes. The poor outcomes of oral cancer are primarily because: (1) most patients are diagnosed at a late stage since the molecular changes that put patients at risk of neoplasia often do not give rise to clinically visible lesions, and (2) a large fraction of patients treated for oral cancer develop subsequent cancers because areas of field cancerization persist following treatment and are not clinically visible.
The goal of this project is to validate the ability of multimodal optical imaging to improve early detection and to determine whether risk-related optical markers (RROMs) can be used to predict the likelihood of malignant progression. To accomplish this, we are conducting longitudinal studies in patients with oral lesions using the PS3 widefield autofluorescence system and the high resolution microendoscope (HRME) with automated algorithms to detect high-grade dysplasia and cancer. We hypothesize that the microscopic information provided by the HRME can reduce false positive diagnoses by autofluorescence. We have also established a transgenic mouse model of oral carcinogenesis to observe RROMs and molecular biomarkers over a timecourse in order to understand how changes in these markers can inform oral premalignant lesion risk assessment.
Publications:
Yang EC, Tan MT, Schwarz RA, Richards-Kortum RR, Gillenwater AM, and Vigneswaran N. Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: current limitations and future directions. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. June 2018. DOI: 10.1016/j.oooo.2018.02.020.
Yang EC, Schwarz RA, Lang AK, Bass NE, Badaoui H, Vohra IS, Cherry KD, Williams MD, Gillenwater AM, Vigneswaran N, and Richards-Kortum RR. In Vivo Multimodal Optical Imaging: Improved Detection of Oral Dysplasia in Low-Risk Oral Mucosal Lesions. Cancer Prevention Research. June 2018. DOI: 10.1158/1940-6207.CAPR-18-0032.
Quang T, Tran EQ, Schwarz RA, Williams MD, Vigneswaran N, Gillenwater AM, and Richards-Kortum RR. Prospective Evaluation of Multi-model Optical Imaging with Automated Image Analysis to Detect Oral Neoplasia In Vivo. Cancer Prevention Research. August 2017. DOI: 10.1158/1940-6207.CAPR-18-0032.
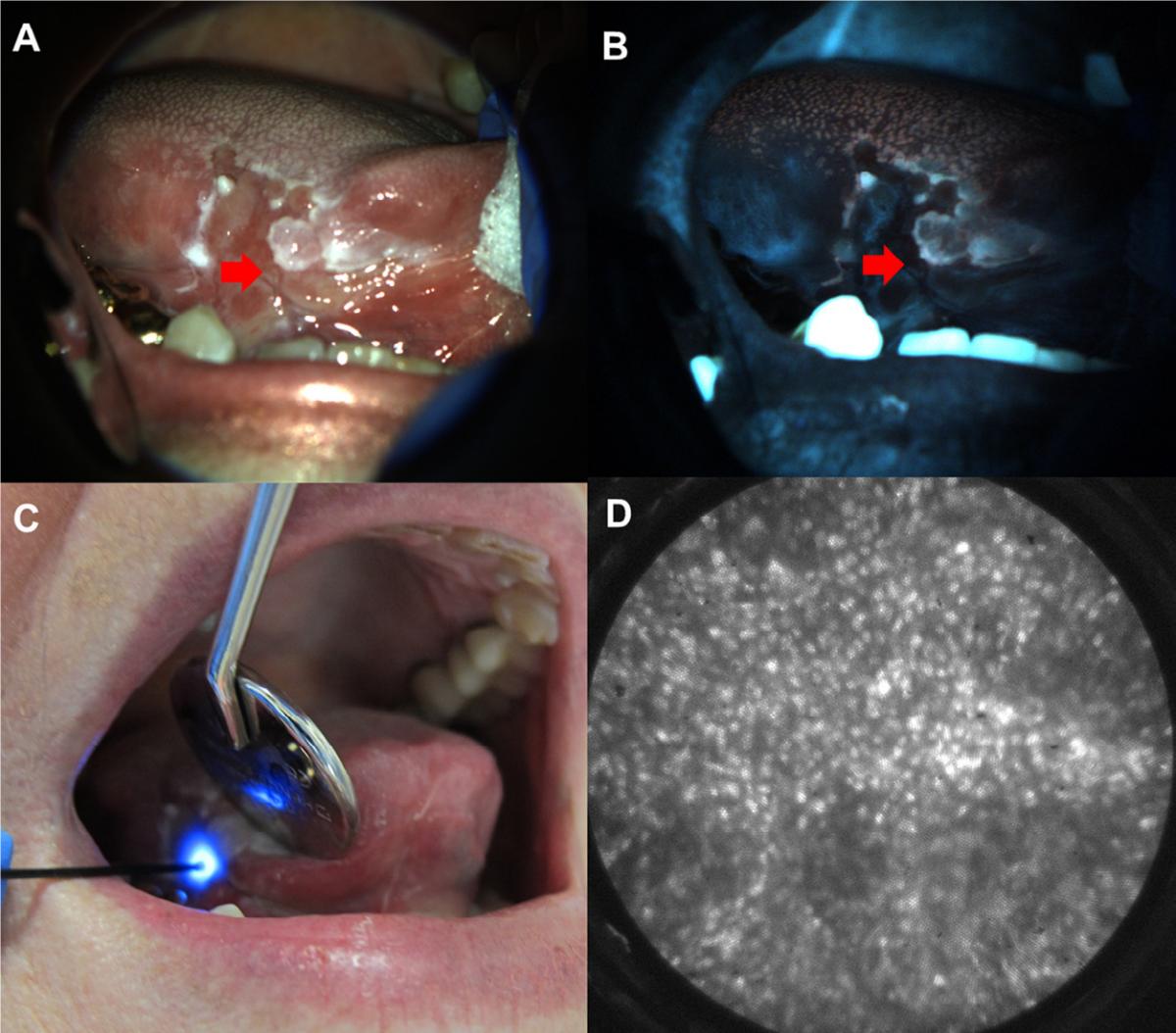
Figure from Yang et al., OOOO (2018): Fig. 3. Multimodal imaging example. White light (A) and autofluorescence image (B) of red and white ulcerated lesion on the right lateral tongue in a 75-year-old female presenting at the University of Texas School of Dentistry. Site imaged with HRME (red arrow) showed loss of fluorescence with elevated normalized red-green ratio. (C) HRME probe on imaged site. (D) HRME image at site showed enlarged, crowded nuclei. Biopsy of the site was performed, and pathologic diagnosis was invasive OSCC.
